Reseach Projects
How memory B cell develop in germinal center?
Upon encountering antigens through B-cell receptor (BCR), B cells are activated and, if the antigen contains protein, take up the antigen and present the antigen-derived peptide on MHC class II to cognate helper T (Th) cells. In turn, Th cells stimulate the B cells through CD40-ligand (CD40L) and cytokines such as IL-4 and IL-21 to facilitate their proliferation and switching of the BCR isotype from IgM/IgD to IgG, IgA or IgE (class-switching). Then some of these B cells differentiate into short-lived plasma cells and move to the extrafollicular region, while others further proliferate and form germinal centers (GC) in the B-cell follicles. In the GC, B cells undergo somatic hypermutation (SHM) of their immunoglobulin (Ig) V region genes to diversify the Ig repertoire. Among the diversified GC B cells, those expressing BCR (typically of IgG classes) that binds to the immunized antigen with high affinity are selected, and differentiate into memory B cells or long-lived plasma cells (LLPCs), both contributing to the long lasting humoral immunological memory.
GC is a characteristic structure that arises in the secondary lymphoid tissues a week after immunization of the protein-containing antigens, so called T-dependent (TD) antigens. GC is composed of intensely proliferating antigen-primed B cells (centroblasts), post-cycled B cells (centrocytes), follicular dendritic cells (FDC) and follicular helper T (Tfh) cells. Tfh cells produce IL-21 that is critical for the prolonged expansion of GC-B cells, and IL-4 that induces the class switching to IgG1 and IgE. It is still unknown what factors are necessary to induce SHM of Ig genes in GC B cells. In-vitro stimulation with factors such as CD40L, IL-4 and IL-21, together with antigens, can induce massive proliferation of B cells and expression of activation-induced cytidine deaminase (AID) that is required for SHM and class-switch recombination of Ig genes, but cannot induce SHM, suggesting some missing factors for SHM. It is also unclear how a few high-affinity B cells are selected among vast majority of others in GC, and how the selected B cells differentiate into memory B cells or LLPCs. Although transcription factors Bcl-6 and Blimp1 are known to be necessary for B cells to differentiate into GC B cells and plasma cells, respectively, transcription factors that induce memory B cells are unknown. External stimuli that induce GC-B-cell differentiation into memory B cells or LLPCs also remain unknown.
One of the reasons for the difficulty to elucidate the mechanisms of the GC B-cell development is the lack of appropriate in vitro system that mimics a process of the development. A few years ago, we have established a B-cell culture system using a newly generated feeder cell line that express exogenous CD40L and BAFF, termed 40LB. In this system, splenic naive B cells undergo massive expansion, express GC B-cell markers such as GL7 and Fas, and undergo efficient class-switching either to IgG1 and IgE. Therefore we called these growing B cells ginduced germinal center B (iGB) cellsh. When we cultured B cells with IL-4 for the first 4 days, and then with IL-21 for the next 4 days, the proliferation was logarithmic and cumulative cell number could increase by 10,000 fold or more. Single B cells could be cloned by the same culture procedure. Despite the extensive proliferation, SHM of Vh region gene could not be detected in the iGB cells.
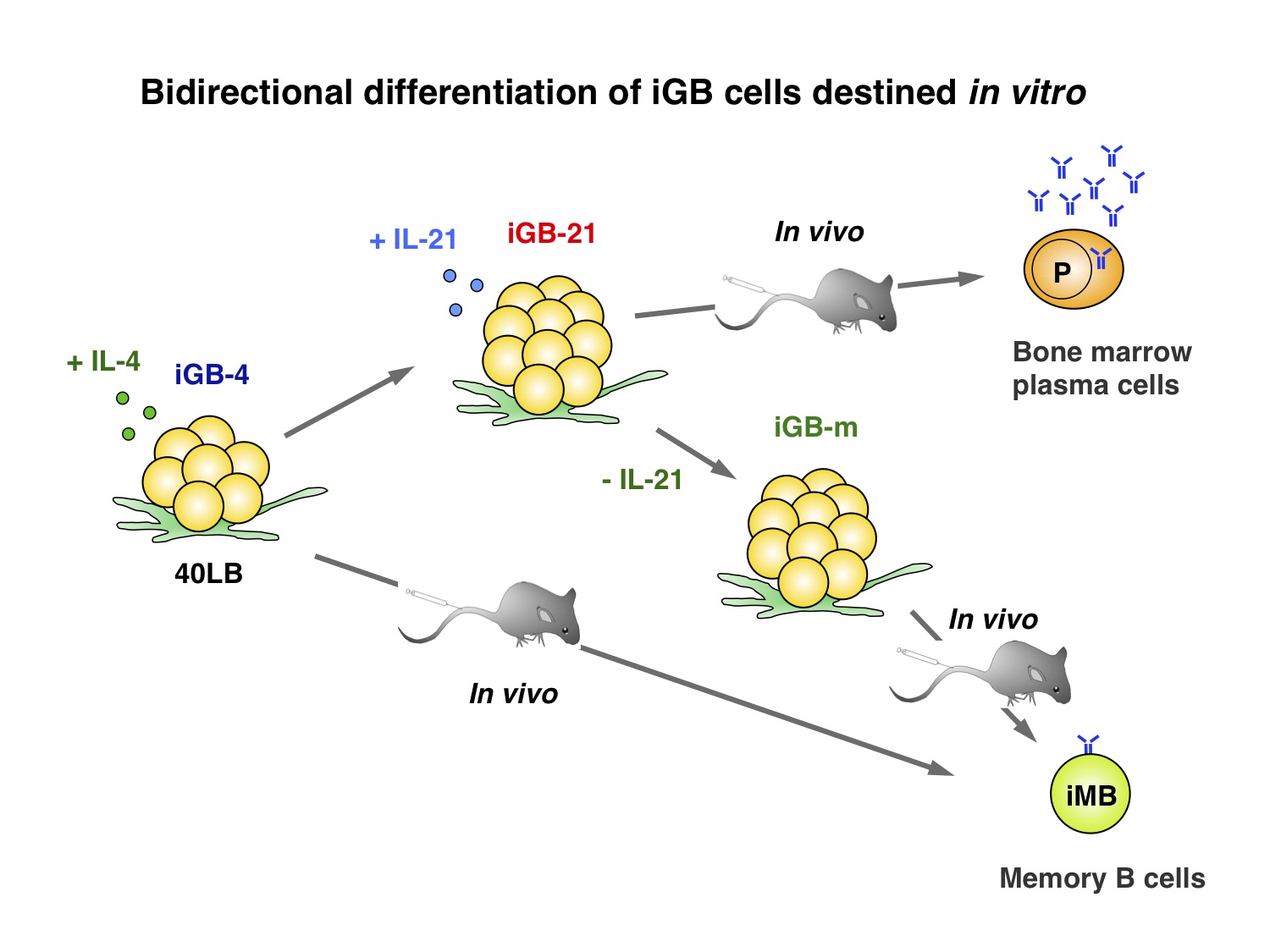
The iGB cells after the first culture with IL-4 differentiate into memory-like B cells, termed ginduced memory B (iMB) cellsh, after transfer into irradiated normal mice. The iMB cells express memory B cell markers, such as CD38 and CD80, but not GC (GL7) or plasma-cell (CD138) markers, and IgM or IgG1, but not IgE. They survive in the recipient mice for as long as 2 months, and, when transferred with cognate Th cells to recipient mice, quickly respond to soluble cognate antigens to produce IgG1 antibodies, indicating iMB cells are functionally equivalent to genuine memory B cells. Interestingly, iGB cells after the secondary culture with IL-21 fail to develop into iMB cells in the recipients, but instead they develop into LLPCs in the bone marrow. This effect of IL-21 is reversible to some extent: the iGB cells cultured for 2 days after withdrawal of IL-21 partly regain the ability to form iMB cells in vivo. (Nojima et al. 2011, Nat. Commun. 2:465) .
Thus, the iGB cell culture system will be useful to elucidate several unsolved questions including regulatory mechanisms of SHM and CSR, affinity selection, the selective fate decision toward memory B cells or LLPCs, requirements for survival of memory B cells and LLPCs, and regulation of memory recall response. Indeed, this system is now used by many researchers all over the world, and the results have begun to be published (Caganova et al. 2013, J. Clin. Invest. 123:5009; Wu et al. 2014, PNAS 111:E4638).
In addition, we addressed a seemingly old but unsolved question, namely, whether memory B cells can respond to T-independent (TI) antigens. We clearly demonstrated that memory B cells induced by a primary TD antigen immunization can elicit recall response to a TI type I antigen (hapten-conjugated LPS) but not to a TI type II antigen (hapten-conjugated Ficoll) carrying the same epitope. We have found that the memory B cells are tolerized by the TI-II antigen, presumably through clonal deletion, and thus do not respond to the tertiary immunization with TD antigen. This tolerance is not due to the lack of Th-cell availability during the recall response, since an antigen having the same epitope on a self-protein (mouse serum albumin) does not cause the tolerance of the memory B cells, but only ignorance. This phenomenon may account for the maintenance of self-tolerance of memory B cells that may have acquired reactivity to self, TI-II-like antigens, during diversification in GC (Haniuda et al. 2011, J. Immunol. 186:5620).

